Biocatalytic Dihydroxylation of Aromatics
In the late 1960's, Gibson and co-worker discovered that soil bacteria are able to convert aromatic compounds to acetate via the corresponding cyclohexadiene diols and catechols. It is possible to isolate intermediates of this biodegradation pathway toobtain synthetically useful metabolites. Cyclohexadiene diols are perfectly suited for the preparation of biologically interesting polyhydroxylated compounds.
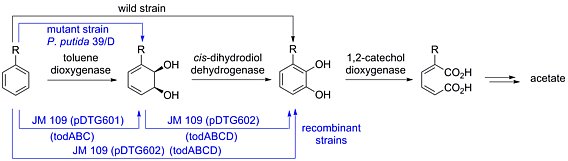
Interestingly, the dihydroxylation of benzoic acid results in the formation of differently substituted dihydroxylation products as outlined below. This process has so far obtained little consideration although the whole cell oxidaion of armoatic compounds presents an environmentally benign method which allows the isolation of value-added chiral synthons from inexpensive and readily available starting materials.
We are interested in the evaluation of the mechansim and the scope of this fascinating whole-cell oxidation and the application of cyclohexadiene diol in the preparation of biologically active polyhydroxylated targets.

Institut für Organische Chemie
Universität Wien
Währinger Strasse 38
1090 Wien
T: +43-1-4277-52166
F: +43-1-4277-9251